Product Name
COVID-19(SARS-CoV-2)IgG/IgM Antibody Test Kit (Colloidal Gold Immunochromatography Assay)
Intended Use
COVID-19(SARS-CoV-2)IgG/IgM Test is used for qualitative detection of novel coronavirus IgG/IgM antibodies in human serum, plasma and whole blood. After infection with the novel coronavirus, the common signs include respiratory symptoms, fever, cough, wheezing and dyspnea, etc. In more severe cases, the infection can lead to pneumonia, severe acute respiratory syndrome, kidney failure and even death. Coronavirus can be expelled from the body through respiratory secretions, transmitted by oral fluids, sneezing, contact, and by airborne droplets.
Principle
COVID-19(SARS-CoV-2)IgG/IgM Test is the colloidal gold labeled rabbit IgG and the novel coronavirus (SARS-CoV-2) antigen, while the nitrocellulose membrane is coated with anti-human IgG and sheep anti-rabbit IgG combined to form a band. The principle of colloidal gold immunochromatography indirect method is used to detect the novel coronavirus (SARS-CoV-2) IgG/IgM antibody in human serum.
Composition
- 1.COVID-19(SARS-CoV-2)IgG/IgM Antibody test cassette(Contains 1 desiccant, 1 pc test device)
- 2.Buffer: 1 bottle
- 3.Instruction: 1 pc
Storage and Expiry Date
- 1.The kit should be stored at room temperature (4-30°C).
- 2.Keep in a dry place away from light.
- 3.Do not freeze the test kit.
- 4.Expiry date: 24 months.
Specimen
- 1.COVID-19(SARS-CoV-2)IgG/IgM Test can be performed used on Whole Blood/ Serum/ Plasma.
- 2.Testing should be performed immediately after specimen collection.
- 3.Use only clear non-hemolyzed specimens.
Test Procedure
Read the instructions carefully before use and bring tests, buffer and specimens were restored to room temperature.
- 1. 10 μl of serum/plasma/whole blood was absorbed and added to the sample hole (S)
- 2. Add 1 drop of sample diluent to the test card sample hole (S).
- 3. Wait for the colored line(s) to appear. The result should be read at 10-15 minutes. Do not interpret the result after 30 minutes.
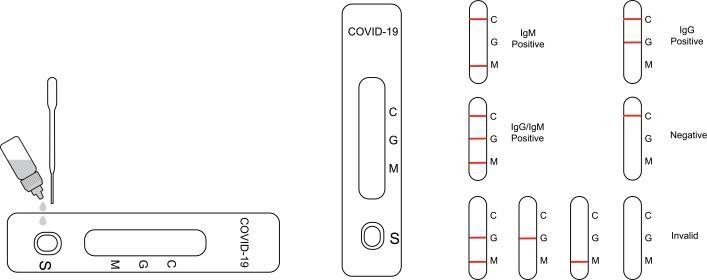
Interpretation of Result
IgG POSITIVE:* Two colored lines appear. One colored line should always appear in the control line region (C) and another line should be in the IgG line region.
IgM POSITIVE:* Two colored lines appear. One colored line should always appear in the control line region (C) and another line should be in the IgM line region.
IgG and IgM POSITIVE:* Three colored lines appear. One colored line should always appear in the control line region (C) and two test lines should be in the IgG line region and IgM lineregion.
*NOTE: The intensity of the color in the test line regions may vary depending on the concentration of COVID-19 antibodies present in the specimen. Therefore, any shade of color in the test line region should be considered positive.
NEGATIVE: One colored line appears in the control line region (C). No line appears in the IgG region and IgM region.
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure an test with a new test. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Limitations of the test
- 1. This product is used for qualitative testing only.
- 2. The test results of this product are for reference only, not as the sole basis for diagnosis and treatment, and should be confirmed in combination with clinical symptoms or other conventional testing methods.
- 3. The negative result may be due to the lower antibody concentration than the analytical sensitivity of the product.
Warnings and precautions
- 1. For in vitro diagnostic use only. Operation should be carried out in strict accordance with the instructions, do not use expired or damaged products.
- 2. Only the diluent in the package can be used, and diluent in different batches cannot be mixed.
- 3. Do not use tap water, purified water and distilled water as negative controls.
- 4. The test device should be used within 1 hour after unsealing. If the ambient temperature is higher than 30°C or more humid, use immediately after tearing.
- 5. If there is no liquid migration in the test window within 30 seconds after adding the detection solution, add another 1 drop of detection solution.
- 6. Pay attention to the possibility of virus infection when collecting specimens, wear disposable gloves, masks, etc., after washing hands.
- 7. The test device is disposable. The test device and specimen after use shall be regarded as medical waste with biological infection risk and properly disposed of according to relevant national regulations.