Details
|
Product Name |
Specimen |
Format |
Catalog No. |
Cut-off(ng/ml) |
Strip width |
|
(CEA)Carcinoembryonic Antigen Test |
Whole Blood/Serum/plasma |
Cassette |
CEA-W02B |
5 |
3.0mm |
|
Cassette |
CEA-W02D |
5 |
4.0mm |
One Step CEA Rapid Screen Test is a rapid direct binding test for the detection of carcinoembryonic antigen in whole blood as an a human tumor marker. The test is based on the principle of sandwich immunoassay for determination of CEA in whole blood. Two monoclonal antibodies are employed to identity CEA specifically. This one step test is very sensitive and only takes about 10-15 minutes. The sensitivity of the test can reach to 5ng/mL.
PRINCIPLE
The CEA Rapid Test Device (Whole Blood/Serum/Plasma) detects human carcinoembryonic antigen (CEA) through visual interpretation of color development on the internal strip. CEA antibodies are immobilized on the test region of the membrane. During testing, the specimen reacts with CEA antibodies conjugated to colored particles and precoated onto the sample pad of the test. The mixture then migrates through the membrane by capillary action, and interacts with reagents on the membrane. If there are sufficient CEA antigens in the specimen, a colored band will form at the test region of the membrane. The presence of this colored band indicates a positive result, while its absence indicates a negative result. The appearance of a colored band at the control region serves as a procedural control, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
SPECIMEN COLLECTION
For whole blood, collect blood into a container with anticoagulant. If the specimen cannot be tested on the day of collection, store the whole blood specimen in a refrigerator. Bring the specimens to room temperature before testing.
TEST PROCEDURE
1. When you are ready to begin testing, open the sealed pouch by tearing along the notch. Remove the test from the pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
2. Place the test device on a clean and level surface. Be sure to label the device with specimen’s ID number.
3. Hold the dropper vertically and transfer one drop of whole blood to the specimen well (S) of the test device.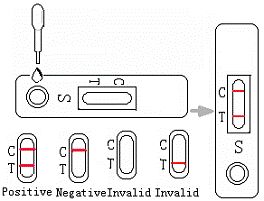
4. Add 50 ul of fresh blood to the Sample Well (for Card) or Sample Pad (for Dipstick),Then add 2 drops (50 ul) of test running buffer into the sample well or sample pad.
5. Wait for the red line(s) to appear. The result should be read in 10-15 minutes. Do not read results after 30 minutes.
INTERPRETATION OF RESULTS
Negative: Only one colored band appears on the control (C) region. No apparent band on the test (T) region.
Positive: In addition to a pink colored control (C) band, a distinct pink colored band will also appear in the test (T) region. This indicates an CEA concentration of more than 5ng/ml. If the test band is equal to or darker than the control band, it indicates that the CEA concentration of specimen has reached to or is greater than 100ng/ml. Please consult your physician to perform a much more detailed exam.
Invalid: If without colored band appears at control region, this is an indication of a possible error in performing the test. The test should be repeated using a new one.
STORAGE AND STABILITY
The test kits can be stored at room temperature (2 to 30 C) in the sealed pouch to the date of expiration. The test kits should be kept away from direct sunlight, moisture and heat.
PRECAUTION
1. For in vitro diagnostic use only.
2. Do not use test kit beyond the expiry date.
3. The test device should not be reused.